“Getting along like oil and water.” This ironic expression describes two incompatible entities that, in fact, do not “get along.” Simply stated, water is denser and more polar than oil; Left undisturbed, the ingression of water into an oil reservoir presents as a separated layer of water at the base of a vessel.
Similarly, if enough air is introduced into an oil reservoir, it too will want to separate itself from the oil. However, since air is less dense, instead of sinking to the bottom like water, it will rise to the surface. Stabilization as a foam layer will depend on additive presence, viscosity, fluid surface tension, container size and other factors. Introduction of air into a lubricant by mechanical agitation is natural and some level of foaming may occur. Therefore, it is worth understanding at which point air becomes a problem.
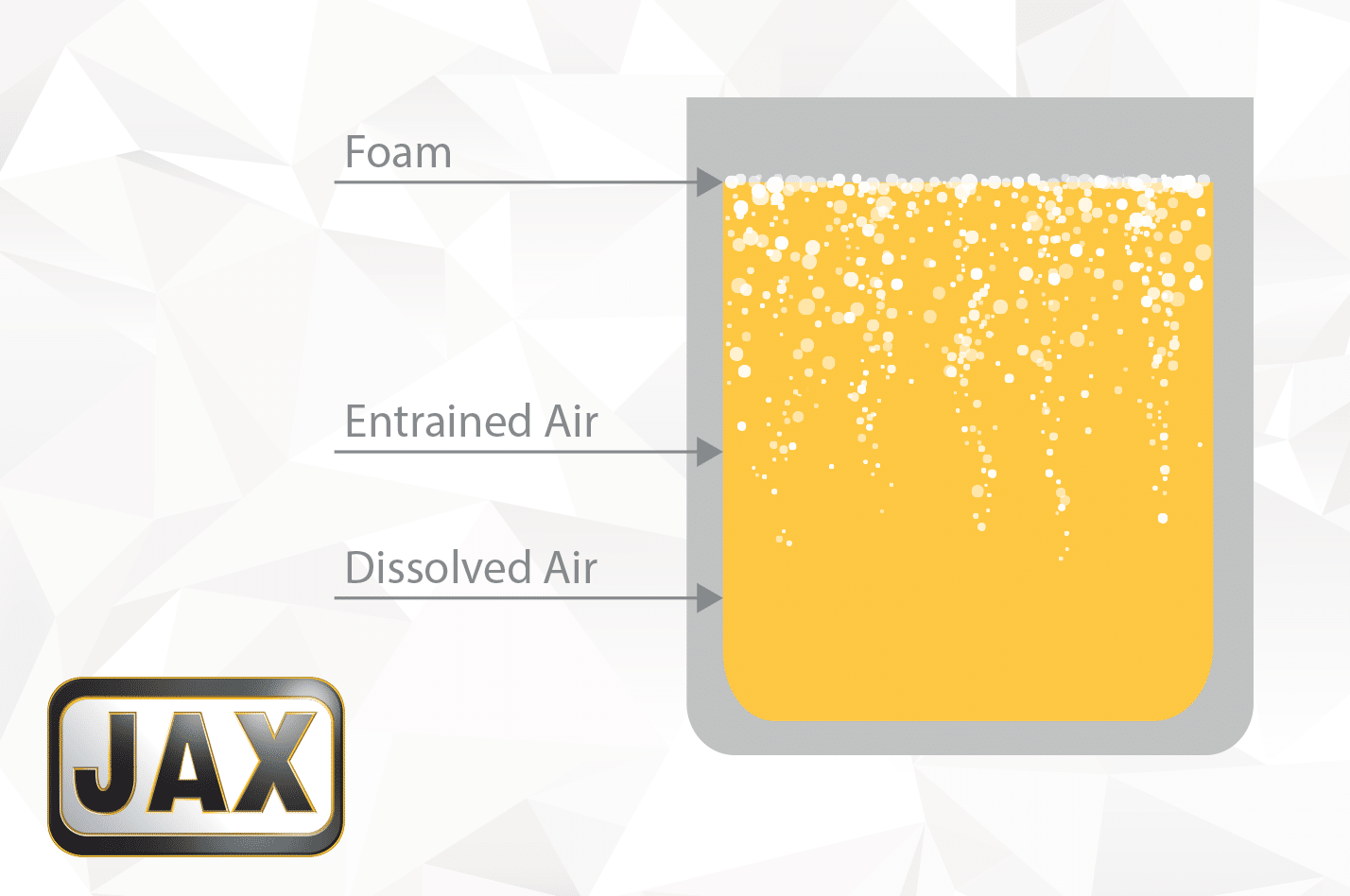
Air Entrainment vs. Foam
Let us start with the obvious: air is all around us; it permeates into every nook and cranny, even becomes entrapped and dissolved in fluids. In every container of oil, microscopic amounts of dissolved air exist – tiny bubbles that are not strong enough to rise against the fluid’s viscosity and escape. Dissolved air can be introduced as bulk fluids are pumped from one vessel to another in production or from the transfer of lubricant from its container into a reservoir. At such small concentrations, dissolved air does not present problems for the end use application.
Air entrainment is the next stage of air introduction. In this case, application related disturbances on the oil reservoir’s surface, force air into the fluid. The higher concentration of insoluble air pockets makes the fluid look more turbid or foggy. Entrained air can occur in splash lubricated gears, fast-moving dip-lubricated chain reservoirs, circulation oil reuptake inlets feeding back into the reservoir or even from “top-off” lubricant poured into a reservoir like a waterfall. The higher the viscosity of the fluid, the slower the entrained air rises to the surface. The larger the bubble, the quicker it rises to the surface.
Eventually, the concentration of low-density air bubbles rises to the surface and forms a frothy layer of foam, distinguishable from the bulk fluid by its lighter color. The layer of entrapped air bubbles must now overcome the fluid’s surface tension before escaping into the reservoir headspace. Ideally, the micro-bubbles of entrained air combine into larger bubbles, the pressure builds until the lamella or bubble walls rupture. Lubricant additives help accelerate this process. The more area the oil surface is exposed to, the quicker the air escapes. So larger reservoirs will degas rather quickly.
Defoamer Additives
Most lubricant formulations will include some means of encouraging air release which fights entrained air or anti-foam which stems the growth/stabilization of the foam layer. There are silicone and non-silicone (polymer) based defoamer additives. These compounds can be thought of as “surface tension modifiers” that provide a weak point for bubbles to break or form larger bubbles. These additives are incompatible with the lubricant by design. If the additives are too compatible, then the functional weak point of the bubble would be less efficient, unable to keep up with continual buildup of entrained air stabilizing as foam.
Even if defoamer additives are included in the formulation of a lubricant, there are application-related variables that limit could limit their effectiveness. For example, if foaming issues are observed following filtration, filter pore size and composition are worth investigating. Filtration of oil with too fine a pore size can sometimes deplete the concentration of antifoam molecules. The material the filter is made of is also worth consideration. Some filter materials have inherent polarity that could capture more of the anti-foam compound. Additionally, contamination with some grease soaps, solids, water or any other polar contaminant will modify the bulk fluid’s surface tension and will counteract the defoamer.
The effects of foaming in lubricants
Imagine a gearbox in a food production facility with foamy oil running down its breathers. If the friction points submerged in the splash lubricated reservoir are sufficiently coated with a lubricating film, the equipment is still protected despite this messy cosmetic external issue. At the same time, if the oil risks contact with finished products or if the oil becomes a slipping hazard, the problem should be mitigated.
If the oil level has dropped such that the friction points are now lubricated by foamy oil, this comes with a host of problems. The air bubbles are gaps in the lubricating film where friction can now occur. In addition, the pressures of the application rapidly destroy these air gaps and could lead to cavitation – rapid compression of the air that delivers a microscopic shock. Over time, this will greatly increase component fatigue.
Laboratory Testing
ASTM D892 is the standard foam test used to assess a lubricant’s foaming characteristics. In the test, 190-200 milliliters of the fluid sample are poured into a 1000mL graduated cylinder. The cylinder is placed in a bath of heated silicone fluid. A compressed air line is coupled to a rod with a standard metal diffusion stone on one end. The rod and stone are submerged in the fluid and allowed to sit for five minutes before the test starts to allow the oil to fill air pockets. The air is allowed to flow at a standard delivery rate for 5 minutes. Air is entrained in fluid and rises to form a foam layer. Following the aeration period, a ten minute degas period is observed to assess how quickly the air is released.
Results are reported with three values. The first value is how much foam formed during aeration. The second value is how much foam was left after the air-release period while the third value is the air-release duration in seconds, with maximum air-release duration being 600 seconds or 10 minutes. Between these three values, we can make conclusions on both foam stability and air release. Most lubricants contain suitable additives that demonstrate satisfactory results for this test. The test finds its greatest use for troubleshooting degradation of the oil as it relates to foaming problems.
*Photos of ASTM D892*
Mitigation
Troubleshooting foaming problems starts with ruling out variables and following the timeline from the when the problem presented. If the same oil is foaming in one piece of equipment but not the other, technicians need to consider what conditions have changed. If the same oil is being used, it should determine the degree of filtration, the age of the equipment, the risk for oil contamination and the last time the oil was changed. Submitting samples for used oil testing will reveal if contamination or degradation is a factor.
Synthetic polyalphaolefins are known to have an inherently lower surface tension and therefore foam less than mineral oils. In theory, one could switch to these synthetics as a foaming mitigation measure, but if subjected to the same contamination, filtration or mechanical problems, the foaming may persist.
Conclusion
Oil foaming is a complex issue. While some base oils are more resistant to foaming than others, almost all lubricants have additives to deal with air introduction. If oil is foaming, usually this is a symptom indicating oil degradation, contamination or even mechanical problems. If you are grappling with foaming, the JAX technical support team can assist in identifying potential sources of the problem.